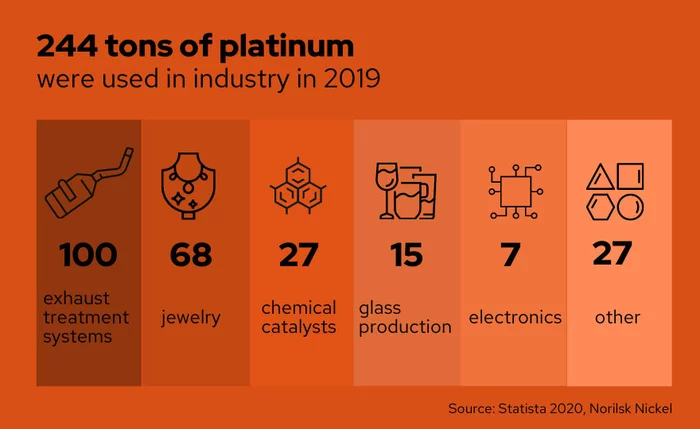
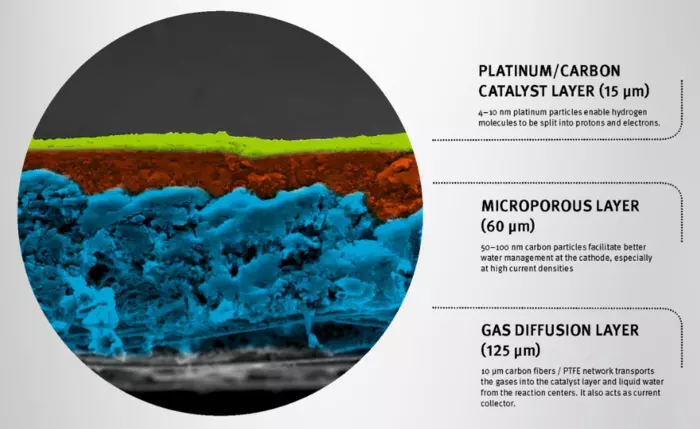
Process Innovation
- The ACHEMA
- For Exhibitors
-
For Visitors
-
Magazine
- Overview
-
Photo Gallery
- Digital Hub
- Engineering
- Events
- Exhibition Grounds
- Industrial and Labour Safety
- Instrumentation, Control and Automation Techniques
- Laboratory and Analytical Techniques
- Literature, Information, Learning and Teaching Aids
- Materials Technology and Testing
- Mechanical Processes
- Pharmaceutical, Packaging and Storage Techniques
- Pumps, Compressors, Valves and Fittings
- Research and Innovation
- Special Show Hydrogen
- Thermal Processes
- Media Library
- Process Innovation
- Pharma Innovation
- Green Innovation
- Lab Innovation
- Digital Innovation
- Hydrogen Innovation
- The Show
- Search
- Press
- Language
-
Login
How do you want to sign in?
As visitor / participant
With your personal myACHEMA account you have access to all extended features of ACHEMA online and to services associated with your participation in our events.
Login with your myACHEMA account to participate in ACHEMA and for access to the ticket shop.
No myACHEMA account yet?
As exhibitor
The ACHEMA Exhibitor Portal provides all information, documents and services for your ACHEMA 2027 participation.
Your login benefits
Save Bookmarks
Add notes
Export entries
Buy tickets
- The ACHEMA
- For Exhibitors
-
For Visitors
-
Magazine
- Overview
-
Photo Gallery
- Digital Hub
- Engineering
- Events
- Exhibition Grounds
- Industrial and Labour Safety
- Instrumentation, Control and Automation Techniques
- Laboratory and Analytical Techniques
- Literature, Information, Learning and Teaching Aids
- Materials Technology and Testing
- Mechanical Processes
- Pharmaceutical, Packaging and Storage Techniques
- Pumps, Compressors, Valves and Fittings
- Research and Innovation
- Special Show Hydrogen
- Thermal Processes
- Media Library
- Process Innovation
- Pharma Innovation
- Green Innovation
- Lab Innovation
- Digital Innovation
- Hydrogen Innovation
- The Show
- Search
- Press
- Language
- Login
How do you want to sign in?
As visitor / participant
With your personal myACHEMA account you have access to all extended features of ACHEMA online and to services associated with your participation in our events.
Login with your myACHEMA account to participate in ACHEMA and for access to the ticket shop.
No myACHEMA account yet?
As exhibitor
The ACHEMA Exhibitor Portal provides all information, documents and services for your ACHEMA 2027 participation.